A solution of h2so4 with a molal concentration of – A solution of H2SO4 with a molal concentration is a crucial component in various industrial and laboratory applications, owing to its unique properties and versatility. This article delves into the intricacies of H2SO4 solutions, exploring their molality, physical and chemical properties, applications, preparation methods, and titration techniques.
Molality, expressed in mol/kg, is a measure of the amount of solute (H2SO4) dissolved in 1 kg of solvent (water). Unlike molarity, which is affected by temperature, molality remains constant regardless of temperature fluctuations. Understanding the concept of molality is essential for accurately preparing and handling H2SO4 solutions.
Molality of H2SO4 Solution

Molality (m) is a measure of the concentration of a solution that expresses the number of moles of solute per kilogram of solvent. It is commonly used in chemistry to describe the concentration of solutions, particularly when the solvent is water.
The unit of molality is mol/kg.
Molality differs from molarity, which is a measure of the concentration of a solution that expresses the number of moles of solute per liter of solution. While molarity is dependent on both the amount of solute and the volume of the solution, molality is only dependent on the amount of solute and the mass of the solvent.
To determine the molality of an H2SO4 solution, the following formula can be used:
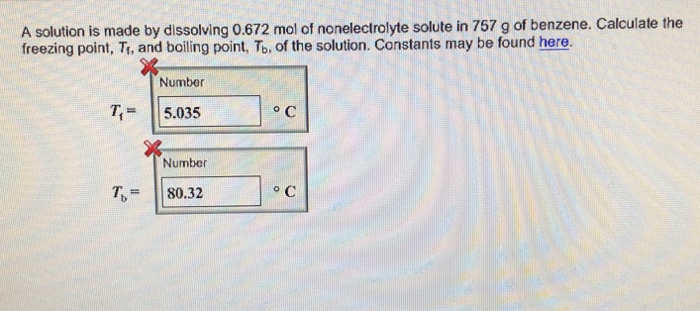
m = moles of H2SO4 / kilograms of water
For example, if a solution contains 0.1 moles of H2SO4 and 1 kg of water, the molality of the solution would be 0.1 mol/kg.
Properties of H2SO4 Solutions

H2SO4 solutions exhibit various physical and chemical properties that depend on their molality. These properties include:
Density
The density of H2SO4 solutions increases with increasing molality. This is because the addition of H2SO4 to water increases the mass of the solution without significantly changing its volume.
Viscosity
The viscosity of H2SO4 solutions also increases with increasing molality. This is because the H2SO4 molecules interact with each other and with water molecules, creating a more viscous solution.
Acidity
H2SO4 is a strong acid, and its solutions are highly acidic. The pH of an H2SO4 solution decreases with increasing molality, indicating a higher concentration of H+ ions.
Hazards
H2SO4 solutions are corrosive and can cause severe burns to the skin and eyes. It is important to handle H2SO4 solutions with care and to wear appropriate personal protective equipment.
Applications of H2SO4 Solutions: A Solution Of H2so4 With A Molal Concentration Of
H2SO4 solutions have a wide range of industrial and laboratory applications, including:
Industrial Applications
- Production of fertilizers
- Oil refining
- Metalworking
- Textile manufacturing
- Water treatment
Laboratory Applications
- Acid-base reactions
- Dehydration reactions
- Electrolyte in batteries
- Cleaning agent
- Etching agent
H2SO4 solutions are also used in the production of other chemicals, such as hydrochloric acid, nitric acid, and sulfuric acid.
Environmental Implications, A solution of h2so4 with a molal concentration of
The production and use of H2SO4 can have environmental implications, such as:
- Air pollution: The release of sulfur dioxide (SO2) during the production of H2SO4 can contribute to acid rain.
- Water pollution: The discharge of H2SO4 into water bodies can lower the pH and harm aquatic life.
- Soil pollution: The use of H2SO4 in fertilizers can lead to soil acidification.
It is important to manage the production and use of H2SO4 in a responsible manner to minimize its environmental impact.
Preparation of H2SO4 Solutions
H2SO4 solutions can be prepared by dissolving concentrated H2SO4 in water. The following steps should be followed to safely prepare and handle H2SO4 solutions:
- Wear appropriate personal protective equipment, including gloves, goggles, and a lab coat.
- Slowly add concentrated H2SO4 to water, not vice versa. This helps to prevent splattering and the release of heat.
- Stir the solution constantly to ensure that the H2SO4 is evenly distributed.
- Allow the solution to cool before using it.
The following table summarizes the preparation procedures for different molalities of H2SO4 solutions:
| Molality (mol/kg) | Volume of concentrated H2SO4 (mL) | Volume of water (mL) |
|---|---|---|
| 0.1 | 3.0 | 1000 |
| 0.5 | 15.0 | 1000 |
| 1.0 | 30.0 | 1000 |
Titration of H2SO4 Solutions

Titration is a technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. In the case of H2SO4 solutions, they can be titrated with standard solutions of NaOH or KOH.
The principles of acid-base titration involve the reaction between an acid and a base to form a salt and water. The equivalence point of the titration is reached when the moles of acid are equal to the moles of base.
At this point, the solution is neutralized, and the pH is 7.
The following steps are involved in titrating an H2SO4 solution:
- Pipette a known volume of the H2SO4 solution into a flask.
- Add a few drops of phenolphthalein indicator to the solution.
- Fill a burette with a standard solution of NaOH or KOH.
- Slowly add the NaOH or KOH solution to the H2SO4 solution while swirling the flask constantly.
- Record the volume of NaOH or KOH solution added until the solution turns a faint pink color.
The concentration of the H2SO4 solution can be calculated using the following formula:
Concentration of H2SO4 = (Volume of NaOH or KOH solution x Molarity of NaOH or KOH solution) / Volume of H2SO4 solution
Questions Often Asked
What is the difference between molality and molarity?
Molality is the amount of solute dissolved in 1 kg of solvent, while molarity is the amount of solute dissolved in 1 liter of solution. Molality is not affected by temperature, whereas molarity is.
What are the hazards associated with handling H2SO4 solutions?
H2SO4 solutions are corrosive and can cause severe burns and eye damage. Proper protective equipment, including gloves, goggles, and a lab coat, should be worn when handling H2SO4 solutions.
How can I safely prepare a solution of H2SO4 with a specific molality?
To safely prepare a solution of H2SO4 with a specific molality, carefully follow established procedures and use appropriate safety equipment. Slowly add concentrated H2SO4 to water while stirring constantly. Never add water to concentrated H2SO4, as this can cause a violent reaction.
